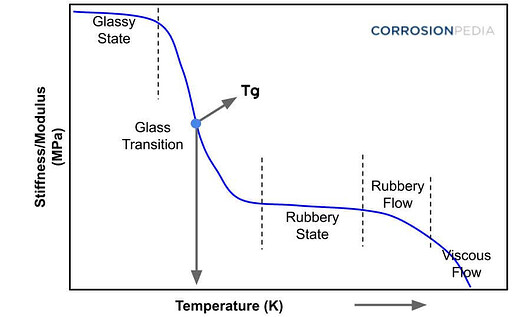
The glass transition temperature is a critical thermal property of polymers. It refers to the temperature range at which a polymer transitions from a hard and relatively brittle “glassy” state into a softer, more flexible “rubbery” state. This transition is not a phase change like melting, but rather a change in the physical properties of the material.
Key aspects of the glass transition temperature include:
- Mechanical Properties: The mechanical properties of a polymer, such as hardness, modulus of elasticity, and strength, change significantly at Tg. Below Tg, the material is harder and more brittle, while above Tg, it becomes softer and more pliable.
- Thermal Expansion: There is usually a noticeable change in the thermal expansion coefficient of the material at Tg. Above Tg, the material tends to expand more with temperature.
- Amorphous vs. Semi-Crystalline Polymers: Tg is most significant in amorphous polymers, where there is a less ordered molecular structure. Semi-crystalline polymers also have a Tg, but it is less pronounced due to their more ordered structure.
- Molecular Mobility: Below Tg, the polymer chains are in a frozen state with very limited mobility. As the temperature increases and crosses Tg, the chains gain more mobility, leading to increased flexibility and ductility.
Understanding the glass transition temperature is crucial in applications where temperature variations are expected. It helps in selecting the right polymer for specific environments, ensuring that the material will perform as required throughout its service temperature range.