These laws define physical quantities like temperature, entropy, and energy.
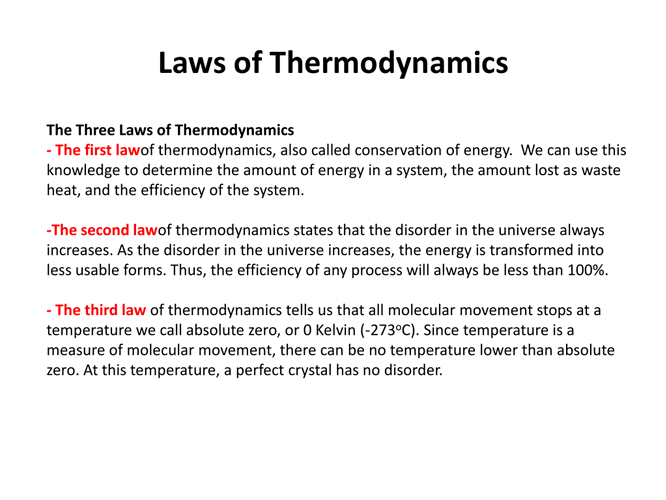
First Law of Thermodynamics: Also known as the law of energy conservation, it states that energy cannot be created or destroyed in an isolated system. The first law is often expressed in the form of the equation
where ΔU is the change in internal energy of the system, Q is the heat added to the system, and W is the work done by the system on its surroundings. This law essentially quantifies the principle of conservation of energy.
The Second Law of Thermodynamics : This law introduces the concept of entropy, a measure of disorder or randomness in a system. The second law states that in an isolated system, the total entropy can never decrease over time.
The Third Law of Thermodynamics : A system’s entropy approaches a constant value as its temperature approaches absolute zero.
More typically in engineering applications we focus on the 1st law of thermodynamics.
Energy cannot be created or destroyed
If you think about it in the context of electro-mechanical parts (pumps, motors, servos, relays, etc.) and computer hardware, no device is 100% efficient - this is why these devices get hot!
The energy transferred between the system and surrounding is summed through the transfer of heat (q) or by the performance of mechanical work (w).