Thermodynamics Subject Matter
The air surrounding us contains water vapor in it. The amount of water vapor present in dry gas is referred as humidity. When a cold bottle comes in contact with the atmosphere we can see the formation of water droplets on the surface of the bottles. It is due to the condensation of water vapor present in the air.
The condensation happens because the water vapor which is relatively at higher temperature (Dry Bulb Temperature) attains its Dew Point (temperature at which vapor condenses at prevailing pressure) as it comes into contact with the cold bottle. As the temperature is declining from Dry Bulb Temperature to Dew Point the amount of vapor present in air starts decreasing by the condensation process and wets the outer surface of the cold bottle.
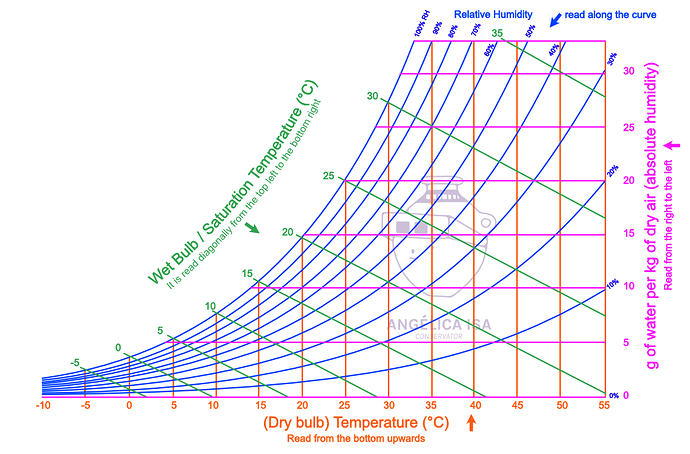
A great visual representation can be seen by reading psychrometric charts (they appear scary, but once you can understand the graph it’s remarkably intuitive!).
The key points are as follows:
- Dry Bulb Temperature: Ambient temperature
- Dew Point: Temperature where water vapor condenses out of the air (100% relative humidity line)
- Absolute Humidity: Actual amount of water that can be “held” by air regardless of temperature
In the case where we go from hot air (i.e. 30 C) to the cold glass (10 C), assuming the same relative humidity of the air (50%), you can see how the absolute humidity of the air changes from ~16 g/kg to ~7 g/kg, and the dew point drops from 21 C to 10 C.
Reference Link: Understanding psychrometric charts and dew points | Angelica Isa