Hint: What happens to water in cold environments?
Glass transition temperature: point below which an amorphous solid (such as glass, polymers, tire rubber, or cotton candy) goes from being ductile to brittle.
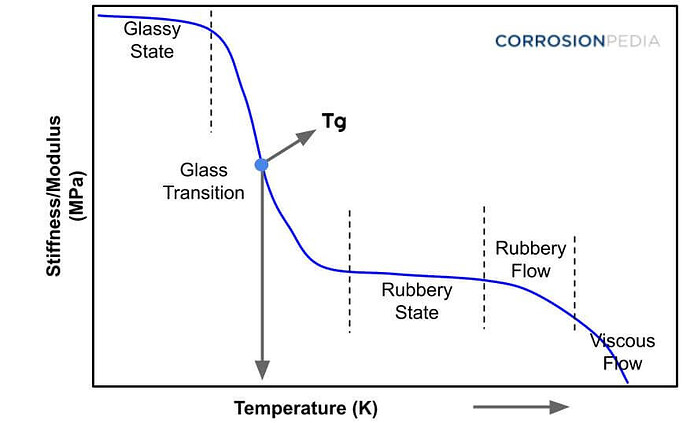
So this is quite an interesting behavior of plastics that I was not aware of! Sharing this graph below for visualization:
A glass transition temperature (Tg ) is the temperature at which a polymer turns from a ductile material to a hard, brittle material.
If we take a take a deep molecular look at plastics, the reason for their ductile behavior in normal room temperature environments is because of the ability of plastic’s long, chain-like molecules stretching out. This is what keeps plastics from brittle behavior as the molecules are free to split past, around, and through each other.
For brittle behavior, if the motion is restricted and the molecules can’t stretch and wrap around each other, areas of localized stress form and when the concentration is too great, cracks propagate and lead to material fracture.
This ability to slip without letting go is the key to ductility, and to avoiding brittle fracture in plastics.
A key factor in the molecules’ ability to slip and slide is temperature. Specifically, there is something called the “glass transition temperature” (Tg), which is the point below which an amorphous solid (such as glass, polymers, tire rubber, or cotton candy) goes from being ductile to brittle.
Many plastics exhibit their transition at everyday temperatures and can be “frozen” into brittleness. One example: polypropylene, an inexpensive material often used in containers, toys, outdoor furniture, and recycling bins has a Tg of between -20 and 0 degrees C, so it can easily lose its molecular mobility and become shatter-prone on a winter day.
Reference: Quotes taken from this MIT post here.